H2 G O2 G H2 0 G Energy Regents
kreativgebiet
Sep 22, 2025 · 6 min read
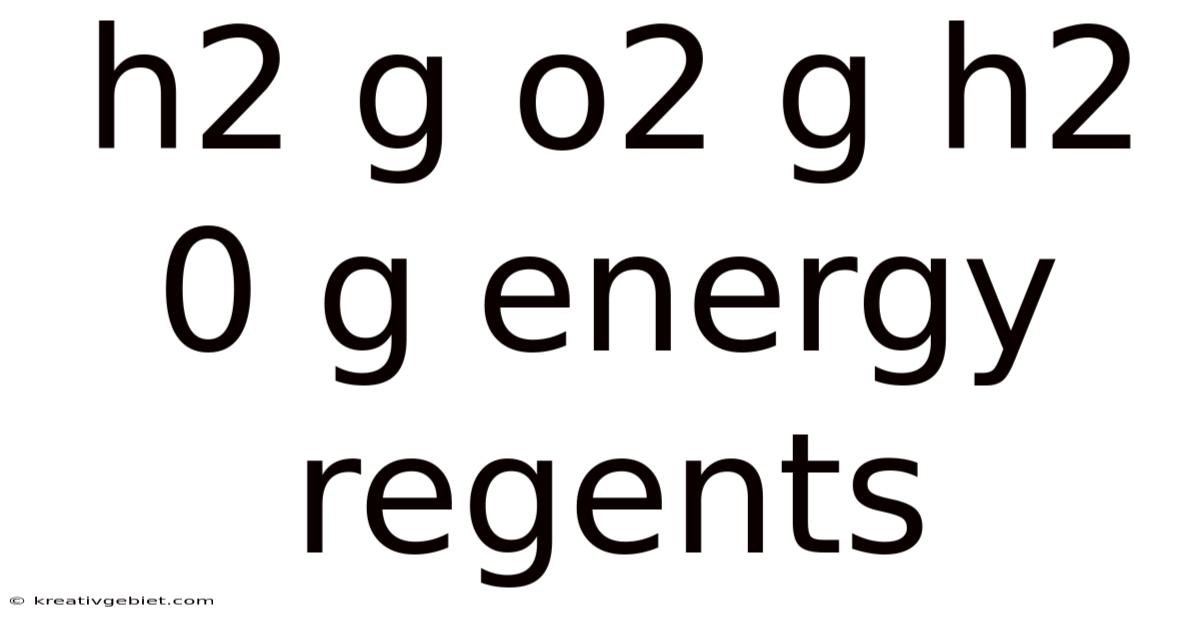
Table of Contents
Understanding the H₂(g) + O₂(g) → H₂O(g) + Energy Reaction: A Regents Chemistry Perspective
The reaction between hydrogen gas and oxygen gas to produce water vapor, releasing energy in the process, is a fundamental concept in chemistry, frequently appearing in Regents Chemistry exams. Understanding this reaction – H₂(g) + O₂(g) → H₂O(g) + Energy – requires delving into its stoichiometry, thermodynamics, and the underlying principles governing chemical bonding and energy transformations. This article will provide a comprehensive explanation, breaking down the concepts in a digestible manner, suitable for Regents level students and beyond.
Introduction: A Closer Look at Combustion
This reaction is a classic example of a combustion reaction, a type of exothermic reaction where a substance reacts rapidly with oxygen, producing heat and light. The rapid release of energy is what makes this reaction so significant, both in terms of its practical applications and its theoretical importance in understanding chemical energetics. The "(g)" notation indicates that all reactants and products are in the gaseous phase. This is crucial because the state of matter influences the reaction's energy changes.
The reaction's equation, as written above, is not yet balanced. Balancing chemical equations is a crucial first step in understanding any chemical process, ensuring that the law of conservation of mass is obeyed. Let's proceed to balance it.
Balancing the Chemical Equation
The unbalanced equation is:
H₂(g) + O₂(g) → H₂O(g)
To balance this, we need equal numbers of each type of atom on both sides of the equation. Let's start with oxygen: There are two oxygen atoms on the left and only one on the right. To balance this, we place a coefficient of 2 in front of H₂O:
H₂(g) + O₂(g) → 2H₂O(g)
Now, let's look at hydrogen: There are two hydrogen atoms on the left and four on the right (2 x 2). To balance this, we place a coefficient of 2 in front of H₂:
2H₂(g) + O₂(g) → 2H₂O(g)
Now the equation is balanced. This means that for every two molecules of hydrogen gas reacting with one molecule of oxygen gas, we produce two molecules of water vapor.
Stoichiometry and Mole Calculations
Stoichiometry allows us to determine the quantitative relationships between reactants and products in a chemical reaction. Using the balanced equation, we can calculate the amount of water produced from a given amount of hydrogen or oxygen, or vice versa. For example:
-
Problem: How many moles of water are produced from the complete combustion of 4 moles of hydrogen gas?
-
Solution: According to the balanced equation, 2 moles of H₂ produce 2 moles of H₂O. Therefore, 4 moles of H₂ will produce 4 moles of H₂O.
-
Problem: How many grams of water are produced from the complete combustion of 10 grams of hydrogen gas?
-
Solution: First, convert grams of H₂ to moles using its molar mass (approximately 2 g/mol). Then, use the mole ratio from the balanced equation (2 moles H₂ : 2 moles H₂O) to find moles of H₂O. Finally, convert moles of H₂O to grams using its molar mass (approximately 18 g/mol).
These types of stoichiometric calculations are common in Regents Chemistry exams, testing your understanding of the relationships between moles, grams, and the coefficients in a balanced chemical equation.
Thermodynamics: Understanding Energy Changes
The "+ Energy" term in the equation indicates that this is an exothermic reaction. Exothermic reactions release energy to their surroundings, usually in the form of heat. This energy release is due to the difference in bond energies between the reactants and the products.
Bond Energies and Enthalpy Change
The reaction involves breaking the bonds in H₂ and O₂ molecules and forming new bonds in H₂O molecules. Breaking bonds requires energy (endothermic process), while forming bonds releases energy (exothermic process). In this specific reaction, the energy released during bond formation in water molecules is significantly greater than the energy required to break the bonds in hydrogen and oxygen, resulting in a net release of energy. This net energy change is represented by the enthalpy change (ΔH), which is negative for exothermic reactions. A negative ΔH value signifies that the reaction releases heat to its surroundings.
Activation Energy
While the overall reaction is exothermic, it still requires an initial input of energy to initiate the reaction. This is the activation energy (Ea), the minimum energy required to break the existing bonds and start the reaction. Once the reaction begins, the energy released during bond formation exceeds the activation energy, leading to a continuous release of energy.
Reaction Rate and Factors Affecting It
Several factors influence the rate of this reaction, including:
-
Concentration of reactants: Higher concentrations of hydrogen and oxygen lead to more frequent collisions between molecules, increasing the reaction rate.
-
Temperature: Increasing the temperature increases the kinetic energy of the molecules, leading to more frequent and energetic collisions, and thus a faster reaction rate.
-
Surface area (if applicable): While this reaction occurs in the gas phase, if the reaction were to involve solid catalysts, increasing the surface area would increase the reaction rate.
-
Presence of a catalyst: Catalysts lower the activation energy of the reaction, making it easier for the reaction to proceed and thus increasing the rate.
Applications of the Reaction
The reaction between hydrogen and oxygen has numerous applications, including:
-
Fuel cells: Hydrogen fuel cells use this reaction to generate electricity, producing only water as a byproduct. This makes it a clean and efficient energy source.
-
Rocket propulsion: The high energy output of this reaction makes it suitable for rocket propulsion systems.
-
Welding: The intense heat produced by the reaction is utilized in oxyhydrogen welding.
Frequently Asked Questions (FAQ)
Q: Is this reaction spontaneous?
A: Yes, under standard conditions, this reaction is spontaneous. The negative ΔH (exothermic) and positive ΔS (increase in entropy due to the formation of more molecules) contribute to a negative Gibbs Free Energy (ΔG), indicating spontaneity.
Q: What are the safety precautions associated with this reaction?
A: Hydrogen gas is highly flammable and explosive, particularly when mixed with oxygen. The reaction should always be conducted under controlled conditions with appropriate safety measures.
Q: Can this reaction be reversed?
A: Yes, through electrolysis, water can be decomposed into hydrogen and oxygen gas. This is an endothermic process requiring an external energy source.
Q: What is the difference between the combustion of hydrogen to form water vapor versus liquid water?
A: The main difference lies in the heat released. The combustion to form liquid water releases more heat than the combustion to form water vapor because the condensation of water vapor releases additional energy.
Conclusion: A Cornerstone of Chemistry
The reaction between hydrogen and oxygen to form water is a fundamental chemical process with significant implications across various fields. Understanding its stoichiometry, thermodynamics, and the factors affecting its rate is essential for comprehending many core concepts in chemistry. This reaction serves as a excellent example of exothermic reactions, combustion processes, and the application of chemical principles to practical applications. Mastering this reaction and its related concepts will provide a strong foundation for further studies in chemistry, particularly in preparation for Regents level exams and beyond. The ability to balance equations, perform stoichiometric calculations, and understand energy changes will be crucial for success.
Latest Posts
Latest Posts
-
Experiment 38 Pre Laboratory Assignment
Sep 22, 2025
-
Silver Ions React With Thiocyanate Ions As Follows
Sep 22, 2025
-
Researchers Investigated The Possible Beneficial Effect
Sep 22, 2025
-
Sec Security Bid Response Proposal Assignment
Sep 22, 2025
-
Which Structure Is Highlighted Chegg
Sep 22, 2025
Related Post
Thank you for visiting our website which covers about H2 G O2 G H2 0 G Energy Regents . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.